February 25, 2021
Currently (as of February 22, 2021), the U.S. Food and Drug Administration (FDA) has approved two COVID-19 vaccines with Emergency Use Authorization (EUA) status: Moderna and Pfizer-BioNTech. These two vaccines insert mRNA into the body that then enters human cells. This mRNA stimulates human cells to manufacture large amounts of the protein that forms the spikes on the SARS-CoV-2 virus. Those protein particles are seen as “foreign” to the body (antigens) and stimulate the human immune system to manufacture antibodies to the SARS-CoV-2 virus spikes. The result is that the body is full of SARS-CoV-2 antibodies before the vaccinated patient is exposed to the actual virus. The antibodies neutralize future waves of SARS-CoV-2 virus particles, and the vaccinated patient stays healthy.1
How will these vaccines impact current COVID-19 testing?
- Antibody (blood sample) testing: The antibody test can now be used to:
- Document that a past COVID-19 infection occurred and stimulated SARS-CoV-2 antibodies.
- Substantiate that an adequate antibody response occurred via vaccination to protect the vaccinated patient from the SARS-CoV-2 virus. After a successful vaccination, COVID-19 antibody test results will be positive.
- PCR (nasal swab) viral testing: Vaccinations with the Moderna and Pfizer-BioNTech mRNA vaccines will not impact the accuracy or use of PCR testing.²
How the Next Vaccines Work
Two other COVID-19 vaccines manufactured by Oxford- AstraZeneca³ and Johnson & Johnson⁴ have just recently been FDA approved for EUA. Instead of using mRNA, these vaccines use an adenovirus—a type of virus that causes the common cold—that has been made unable to replicate. The adenovirus carries a gene from the coronavirus into human cells, which then produce the coronavirus spike protein, but not the coronavirus itself. These spike proteins stimulate the vaccinated patient’s immune system to make antibodies to the coronavirus spike proteins.
How will these two vaccines impact COVID-19 tests?³,⁴
- Antibody (blood sample) testing: COVID-19 antibody test results will be positive after a successful vaccination series with either of the two new vaccines because the vaccinated patient will manufacture the antibody.
- PCR (nasal swab) viral testing: These new vaccine types do insert some coronavirus genomic material into the body, and that might make one think they could interfere with the PCR test. These new vaccines however, do not impact the PCR test².
- The genetic material is injected into the arm and is not located on the mucous membranes of the nasopharynx where the PCR test swab is inserted to obtain a sample.
- The circulating genetic material in the vaccine is made of fractional parts of the coronavirus genome and is at a concentration in the body that is far below the threshold necessary to show as a PCR positive test result for “Virus detected.”
What to Look For in COVID-19 Testing Trends
The CV19 Lab Testing Dashboard powered by hc1 will continue to report COVID-19 PCR prevalence rates in both a county and hyper-local manner that will not be impacted by those individuals that receive the vaccine for preventing COVID-19. As more Americans are vaccinated, we expect that the prevalence rates of positive PCR tests, reported as Local Risk Index (LRI), will decline across the country.
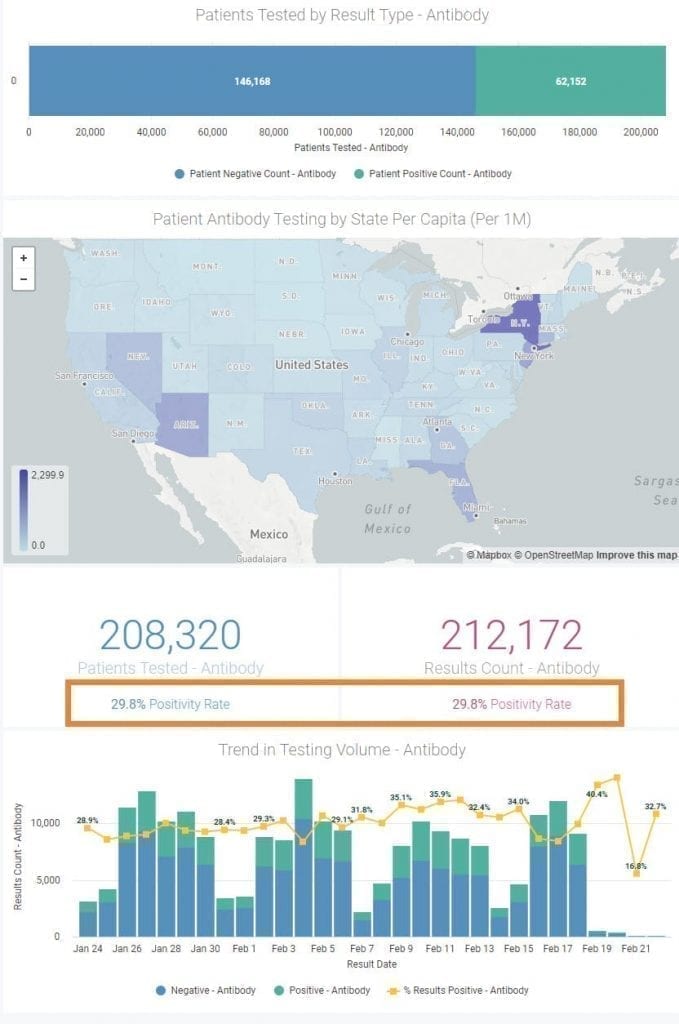
Our reporting of COVID-19 antibody testing will now reflect not only the number of individuals with a past resolved infection who have mounted an internal protective antibody response, but also those who have a successful response to the vaccine two-shot series and have been tested. In effect, the Antibody testing side of the dashboard will now be a gauge of overall “herd immunity” in your local community. When the Positivity Rate gets to 70 or 80%, perhaps the immediate threat of the COVID-19 pandemic will be behind us.
References
- CDC and Infectious Diseases Society of America (IDSA). COVID-19 Real-Time Learning Network Vaccines FAQ. Updated February 19, 2021. https://www.idsociety.org/covid-19-real-time-learning-network/vaccines/vaccines-information–faq
- Corum, Jonathan and Zimmer, Carl. How the Oxford-AstraZeneca Vaccine Works. The New York Times, updated February 3, 2021. https://www.nytimes.com/interactive/2020/health/oxford-astrazeneca-covid-19-vaccine.html
- Corum, Jonathan and Zimmer, Carl. How the Johnson & Johnson Vaccine Works. The New York Times, updated February 3, 2021. https://www.nytimes.com/interactive/2020/health/johnson-johnson-covid-19-vaccine.html